
***RECALL ALERT*** Boston Scientific has recalled roughly 203,000 pacemaker devices in its ACCOLADE family of products. If you or a loved one has been injured by a recalled pacemaker, contact our Boston Scientific pacemaker lawyers today for a free consultation.
Even if you are not sure if your device has been recalled, give us a call so we can help you find out.
The crux of the Boston Scientific recall is that a manufacturing defect causes high battery impedance levels, which in turn causes the pacemaker to shut down.
When the pacemaker device shuts down, it can cause life-threatening injuries or even death.
If you think you may have suffered an injury, do not delay. Contact our experienced Boston Scientific pacemaker recall injury attorneys for a free case evaluation.
Our biggest concern is making sure you get the justice and the compensation that you deserve.
Please don’t hesitate to reach out to us 24/7: 866-837-1010.
What Is A Boston Scientific ACCOLADE Pacemaker?
Boston Scientific’s ACCOLADE line of pacemakers are designed to regulate adbnormal heart rythm in patients.
Typically, pacemakers are used to treat arrhythmias, which is a condition where the heart cannot pump enough blood to the body.
The most common reason people get a pacemaker is their heart beats too slowly (bradycardia), or it pauses, which causes fainting.
In less common cases, pacemakers are used to regulate heartbeat that is too fast (tachycardia).
These devices function by sensing the heart’s rythym and then sending electrical pulses to the heart to control the heart’s rythym.
If a Boston Scientific pacemaker malfunctions, the patient’s heart rythym may not remain in normal levels and adverse cardiac events may occur.
The Boston Scientific Pacemaker (ACCOLADE) Recall
Boston Scientific announced a recall for a subset of its “ACCOLADE Family of Pacemakers”.
In addition, Boston Scientific confirmed that 13% all pacemaker devices made (roughly 203,000 units) before September 2018 are subject to the recall. Frankly, this is a huge number for a medical device recall.
Tragically, Boston Scientific also reported two confirmed deaths of patients that used their defective pacemakers.
Regarding the reason for the recall, what we’ve learned so far is that a manufacturing defect in the device causes high impedance levels in the pacemaker’s battery unit, which in turn causes the device to shut down.
Boston Scientific calls this event going into “safe mode”. But really thats’s just a nice way of saying the device fails.
Unfortunately, when the pacemaker fails, it can cause people to have an adverse cardiac event or death.
Gice us a call to discuss your case. Even if you are not sure what caused you or your loved one’s cardiact event or death, contact our Boston Scientific pacemaker recall lawyers today for a free consultation.
Which Boston Scientific Pacemakers Have Been Recalled?
Specifically, Boston Scientific recalled the following pacemaker models:
- Accolade,
- Proponent,
- Essentio,
- Altrua 2 Standard Life (SL),
- Extended Life (EL), and
- Visionist and Valitude cardiac resynchronization therapy pacemakers.
Additionally, Boston Scientific published “Appendix A”, which is a list a recalled devices by product name and model number:
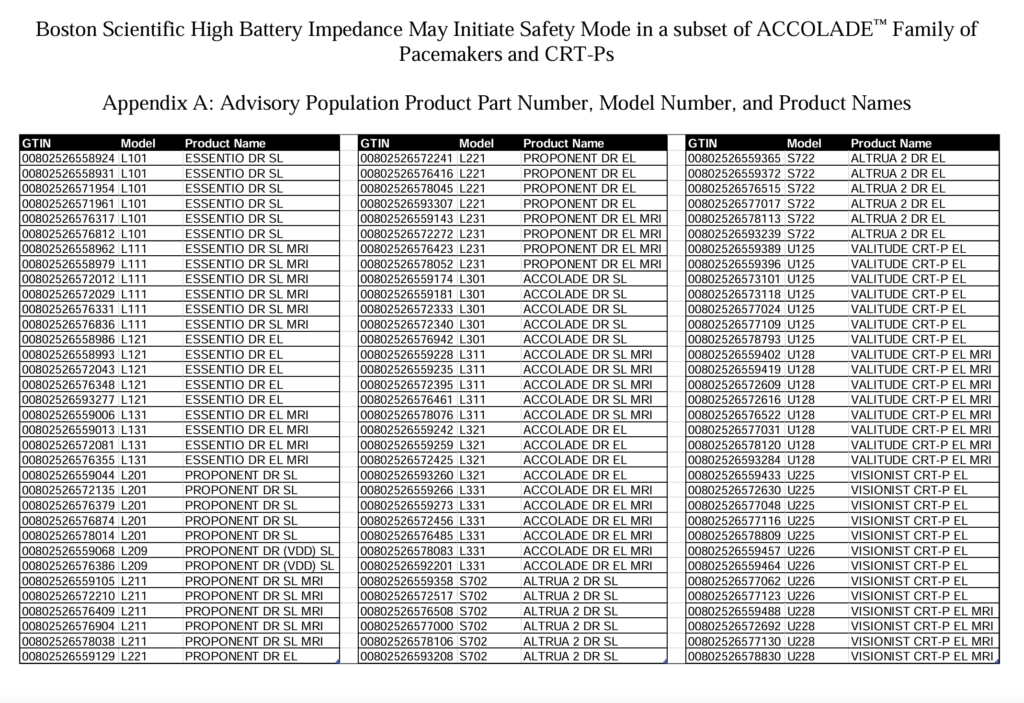
If you need help determining if your device is subject to the recall, give us call today.
Who Qualifies for the Boston Scientific Pacemaker Recall Lawsuit?
Anyone (or a loved one) who has or had an implanted Boston Scientific recalled pacemaker device that was recalled may have a case.
To see if your case qualifies, contact a Boston Scientific recall lawyer today. You can reach us 24/7 at 866-837-1010.
What Can I Recover From A Boston Scientific Pacemaker Lawsuit?
Every case is unique, but people who successfully file product liability lawsuits can recover money for the following damages:
- PAST AND FUTURE MEDICAL BILLS (INCLUDING MEDICATION, HOSPITAL STAYS, AND IN-HOME CARE)
- PAIN AND SUFFERING
- LOST WAGES
- LOSS OF EARNING CAPACITY
- FUNERAL EXPENSES (IN THE VENT OF A LOVED ONE’S DEATH)
- BROADLY SPEAKING, A PLAINTIFF COULD BE ENTITLED TO COMPENSATION FOR ANY PAST AND FUTURE COSTS ASSOCIATED WITH THEIR INJURY
To speak to an experienced Boston Scientific pacemaker attorney about your damages, call us today for a free consultation.
How Much Does It Cost To Hire A Boston Scientific Recall Lawyer?
Fob James Law Firm operates on the contingency-fee-basis. We only get paid a percentage of the recovery if we successfully resolve your case.
Our fees come out of a favorable settlement or jury award, not out of your pocket. If we do not obtain a recovery then you owe us nothing.
To speak with one of our experienced defibrillator lawyers, call us today at 866-837-1010.
How Long Do I Have To File A Boston Scientific Lawsuit?
Every state is different so make sure you consult with an attorney about how long you have to file a lawsuit.
In many states, you generally have two years from the date of discovery of your pacemaker related injury to file a lawsuit. However, some states allow only one year to file a lawsuit.
Please do not assume you have a specific amount of time. Contact our Boston Scientific recall attorneys for a case review.
How Do I Join The Boston Scientific Pacemaker Lawsuit?
It is simple to join the Boston Scientific pacemaker lawsuit. Call us today or submit your contact information via our confidential form. Our attorneys will call you to discuss your case.
It does not matter where you live. We represent victims of defective catheter devices in all 50 states.
Boston Scientific Pacemaker Litigation Updates
Our mass tort attorneys will post important information about the recall and related pacemaker litigation here.
November 3, 2025 – General Overview of the Accolade Recall and Litigation
As of November 2025, the litigation landscape surrounding the Accolade family of pacemakers continues to develop in several key areas:
1. Recall status and regulatory oversight
- In December 2024, Boston Scientific issued a recall of a subset of its Accolade, Proponent, Essentio, Altrua 2 (SL/EL) dual‑chamber pacemakers and Visionist/Valitude CRT‑P devices due to a manufacturing defect in the battery cathode, which can lead the device to permanently enter “Safety Mode.”
- In February 2025 the Food & Drug Administration (FDA) upgraded the recall to a Class I, its most serious level.
- The FDA reports at least 832 injuries and two deaths associated with the malfunctioning devices.
- The affected devices are those built before September 2018 (approximately 13% of the Accolade family).
2. Legal claims and litigation status
Fob James Law Firm is investigating product‑liability claims on behalf of individuals who experienced:
- device failure,
- unexpected entry into Safety Mode,
- need for early device replacement or revision surgery, and
- in some cases catastrophic cardiac events.
Importantly, law‑firms are actively investigating potential claims even if device revision has not yet occurred, advising patients with affected implants to consult counsel regarding their rights.
While no class action specific to the Accolade litigation has been publicly announced (as of this date), we encourage you to monitor Boston Scientific’s communications and the FDA’s ongoing monitoring of the issue.
3. Key legal issues at play
Design/Manufacturing defect: The core allegation is that the battery cathode manufacturing process led to latent elevated impedance, under‑powering the device and causing it to switch to Safety Mode during normal operation. (U.S. Food and Drug Administration)
Failure to warn/monitor: Plaintiffs allege the manufacturer failed to adequately warn about the risk of Safety Mode or design warning and monitoring protocols for the affected cohort.
Medical monitoring and mitigation: Some legal claims focus on whether patients should have had earlier device replacement, or more intensive monitoring after receiving follow‑up advisory communications.
Statute of limitations and eligibility: Because the advisory devices were manufactured before September 2018, eligibility may depend on date of implant, date of discovery of the malfunction, state‑specific limitations, and whether the patient suffered injury or had revision. We advise you seek prompt case evaluation.
4. What’s next and what to watch
- Monitor for any class‑action filing, certification, or mass‑tort consolidation of claims against Boston Scientific.
- Watch for any settlement offers or structured claim‑programs by Boston Scientific or its insurers. Some counsel report early‑stage communications from the manufacturer offering minimal compensation (e.g., reported $2,500 offers) – important for plaintiffs not to accept without legal review.
- Continue to track FDA updates: The FDA is still working with Boston Scientific “to evaluate the potential risk of this issue in all Accolade pacemaker devices and identify additional mitigation strategies as needed.” (U.S. Food and Drug Administration)
- We encourage patients to consult with their cardiologist and legal counsel regarding whether their device falls within the recalled cohort, the monitoring status of their device, and their options for remedial action or claim filing.
5. Document Important Information and Events
- Confirm implant date, model/serial number and whether the device is in the advisory population (built before Sep 2018).
- Document any symptomatic events (e.g., syncope, bradycardia, device revision) and any communications from Boston Scientific or the patient’s physician regarding this advisory.
- Preservation of evidence: medical records, device interrogations, implant/removal operative reports and any manufacturer communications.
April 28, 2025 – Accolade Recall Limited to Pacemakers Implanted Before 2018
We have been getting many calls from prospective clients about their Accolade devices. The recall is for devices implanted before 2018. As a result, we are unable to take cases where the device was implanted in 2018 and beyond. If this changes we will post an update here.
April 4, 2025 – Boston Scientific Allegedly Offering $2500 to Victims
From talking to potential clients we have learned that Boston Scientific is offering Accolade victims a whopping $2500 for their trouble in getting a surgery to explant the defective device and install a new device. We think this “offer” is unacceptable. We encourage anyone that receives the $2500 offer to speak with an attorney before depositing the funds or signing any documents.
February 27, 2025 – FDA Upgrades Accolade Recall to Class I
The FDA upgraded the Accolade recall to a Class I, which is the most serious type. This is a big deal.
So far more than 832 injuries and two deaths have been reported. The FDA did not sugarcoat its reasoning:
This recall invovles the potential need for device explant. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it…
If you have an implanted Accolade pacemaker, we highly recommend that you speak with your cardiologist immediately.
December 16, 2024 – Boston Scientific Recalls ACCOLADE Pacemakers
As discussed above, Boston Scientific announced a massive recall in connection with its ACCOLADE line of pacemakers. You can read the recall announcement here. We will post additional updates as new information becomes available
Related Content: Read about the Boston Scientific Defibrillator (S-ICD) litigation
Contact a Boston Scientific Accolade Lawyer Near Me
At Fob James Law Firm, our job is to help you to the best of our ability and fight for you. If you’ve suffered an injury as a result of a Boston Scientific pacemaker device, you should contact our attorneys immediately.
We can determine if you are eligible to file a lawsuit or not. It won’t cost you anything to speak with us. You will never pay us anything until we successfully settle or win your case in court.
Our nationwide Boston Scientific Recall Lawyers are experienced, dedicated, and they truly care about you. We treat all of our clients exactly how we would want our own family members to be treated.
Contact us right now at 866-837-1010 or set up a free case evaluation so we can help you.